Genuine AIS Anode
The heart of every AIS chlorine generator.
As a leading water disinfection company AIS insists on high standards, particularly when it comes to the technology at the heart of each one of its chlorine generators. When AIS could not find an anode supplier to meet its exacting standards, the company took matters into its own hands and decided to manufacture its own.
In 1998 the first batch of Genuine AIS Anodes™ was born. Today AIS produces thousands of anodes each year at one of its three production facilities in Brisbane, Australia. Genuine AIS Anodes™ begin their life as a plate of pure, high-quality titanium. Titanium has a unique strength-to weight ratio, resists corrosion and is non-allergenic. Each pure titanium plate undergoes a multi-stage cleaning and etching process which allows it to bond with AIS’ unique ruthenium compound-based catalytic coating (which is painstakingly applied and cured, one microscopically thin coat at a time).
The curing process involves repeated baking, to temperatures that would melt lesser metals. Over many weeks, the catalytic coating is turned into a ceramic-like material that is much more efficient in chlorine generation than the original titanium beneath it. Despite being covered in dozens of layers, the total width of the catalytic coat on some AIS Anodes™ is less than a fifth of the width of a strand of human hair. Genuine AIS Anodes’™ proprietary coating increases the efficiency of the electrolysis process along with the life of the electrodes. The finished anodes are then cut to size and assembled by AIS’ skilled and experienced production team to create the electrolytic cells that facilitate the electrolysis process at the heart of all AIS chlorinators.
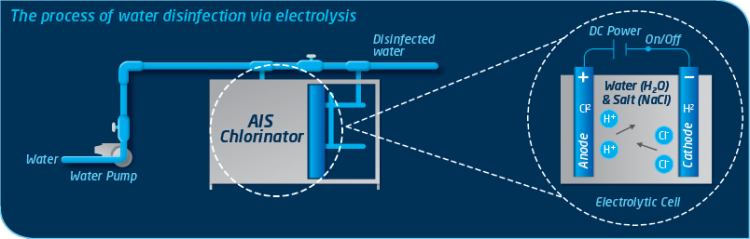
Inside each electrolytic cell an electrical current passes between two electrodes through an electrolyte (water containing minerals like sodium chloride). Hydrogen ions move to the cathode and turn into hydrogen. Chloride ions move to the anode and turn into chlorine. Meanwhile sodium and hydroxide ions get left behind and stay in the solution. This provides all the necessary ingredients for the automatic formation of Hypocholous Acid (commonly referred to as Chlorine), an effective and proven water disinfectant. This process occurs not only within the electrolytic cell but inside the treated water itself, meaning a stop to the endless cycle of traditional chemical dosing forever.